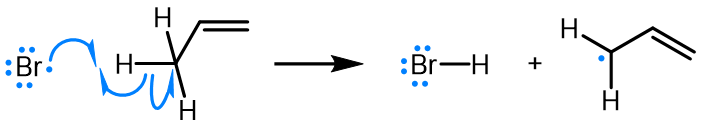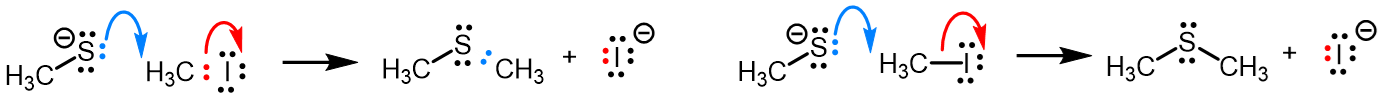Mechanism Problems: Draw Curved Arrows
In these problems you will be asked to draw chemically reasonable curved (curly) arrows to depict the electron movement in the transformations. (Problems using the 'dotted line' convention, in which the formation of new bonds must be indicated by drawing dotted lines between the atoms, are also available.)
The mechanism of a reaction is the sequence of 'elementary' steps in the reaction, including details of the bonds are formed and/or broken in each step. Understanding mechanisms is key to understanding the reactions of organic compounds, and is essential to being able to use those reactions to make useful compounds.
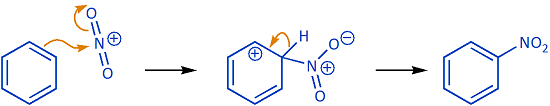
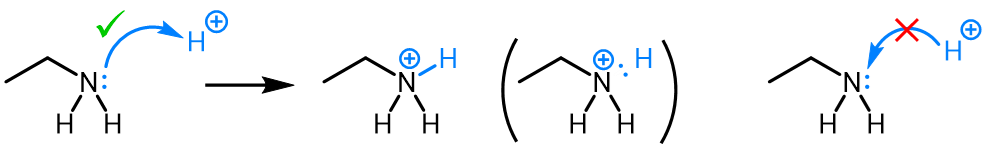
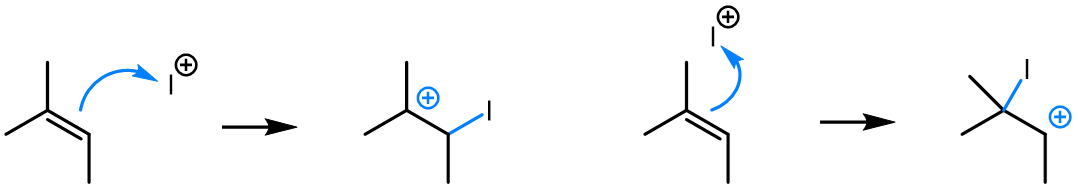
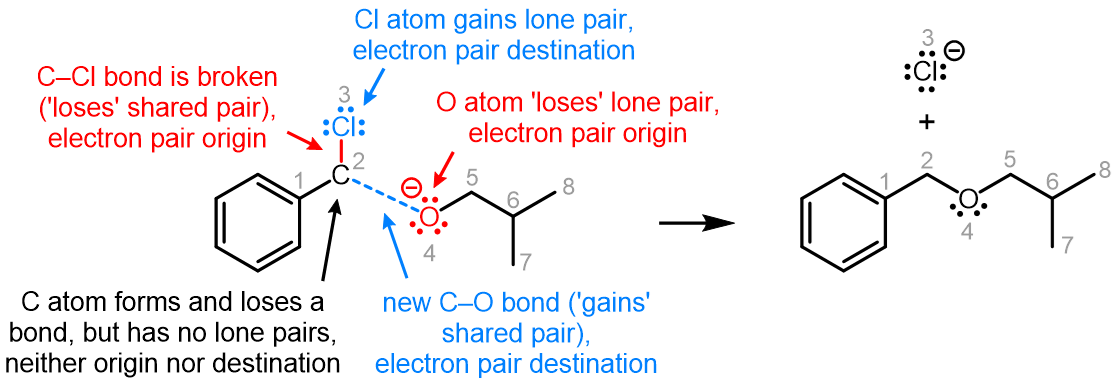
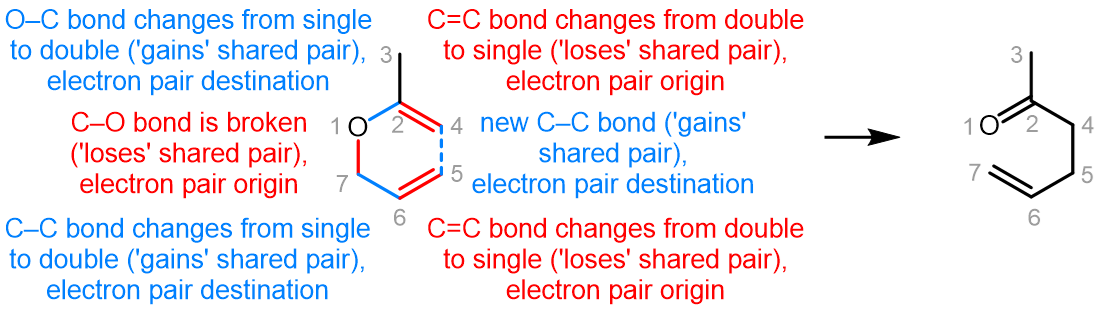
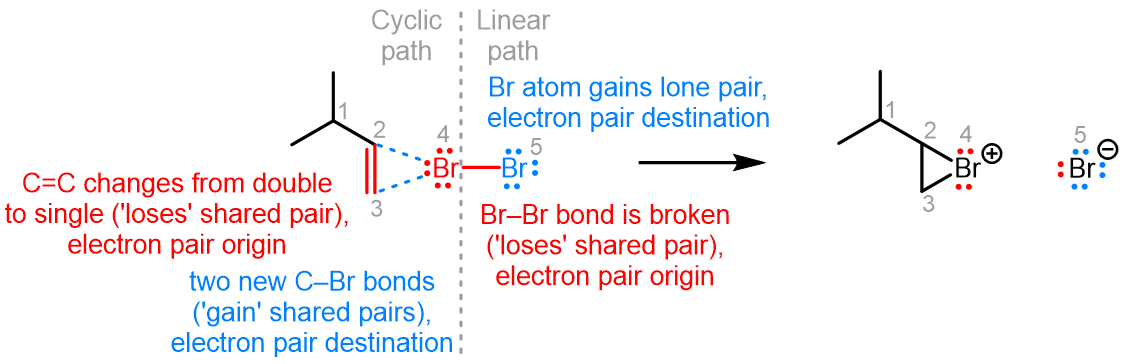
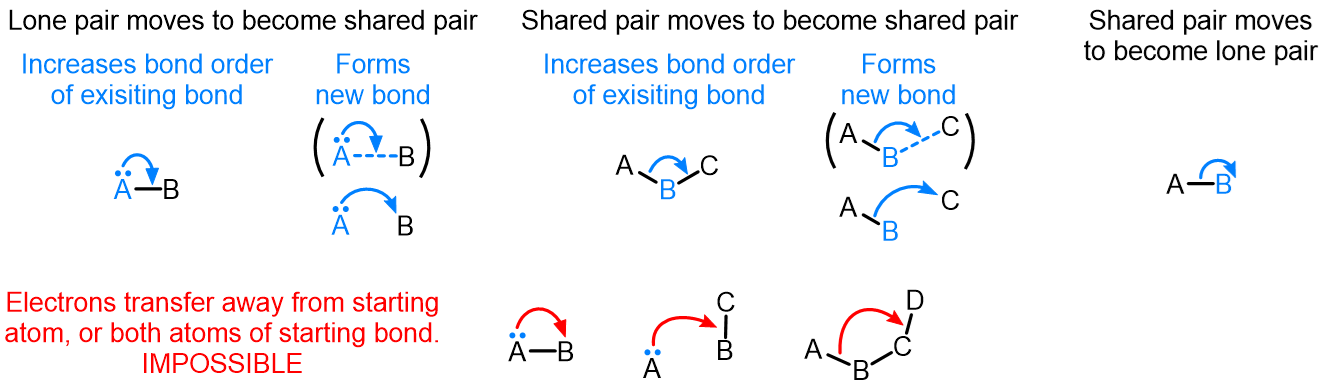
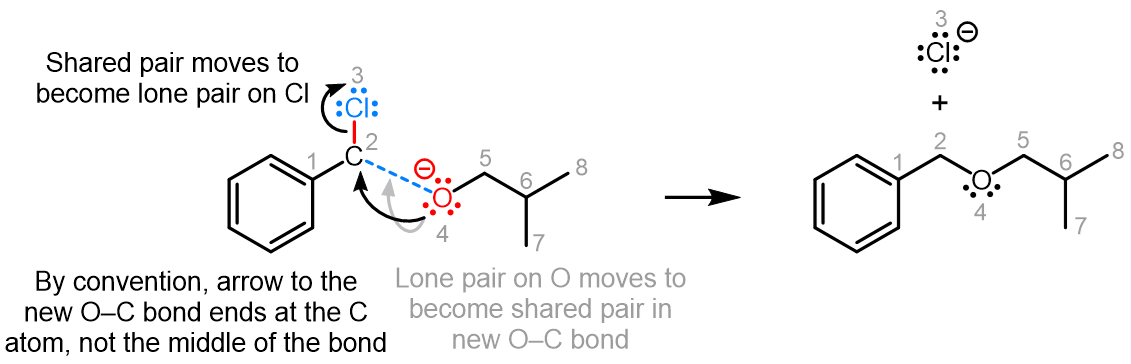
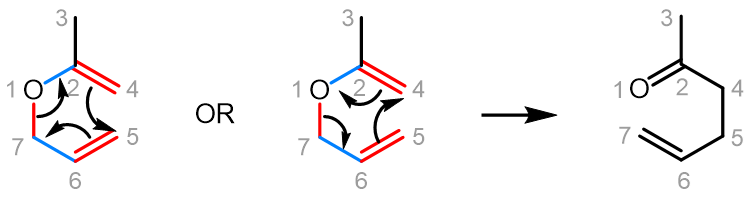
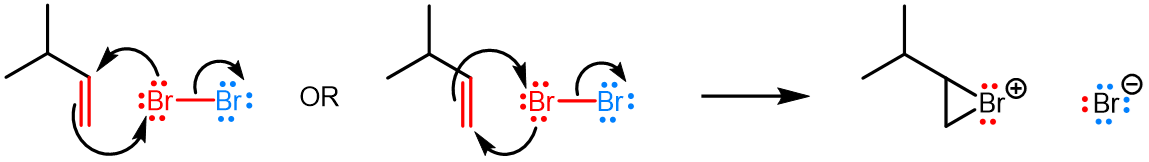
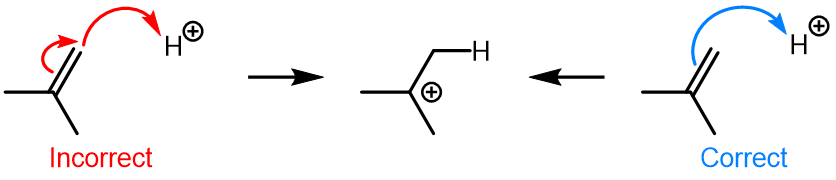
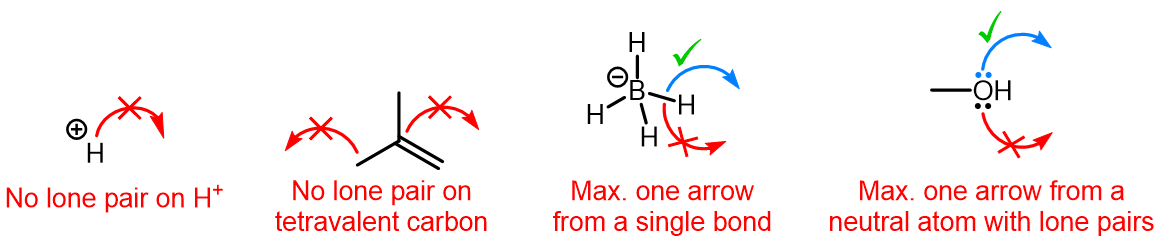
Curved (curly) arrows, aka 'electron pushers', are used to represent the movement of electrons in the bond forming and breaking events in the mechanism. The problems on this page do not require you to predict mechanisms, they provide practice in the correct use of curved arrows to depict electron movements. Starting materials, intermediates and products for common reactions are provided and you are asked to draw the curved arrows that represent the movements of electrons that account for the bonding changes in the transformations.
Note that 'correct' curved arrow mechanisms are provisional, further research may show that some of them are incomplete or wrong. Moreover, they only show reasonable pathways of electron movement, and do not include any information about other aspects of the mechanism. It is best to think of curved arrow 'mechanisms' as greatly simplified models that are nevertheless extremely useful because they help chemists to understand key aspects of the reactions.
On This Page:
You will be given the starting materials, intermediates and products for organic reactions and can practise drawing the curved (curly) arrow mechanisms for the transformations.
Many common mechanisms are included, and they are organised by type (e.g. 'acyl substitution').
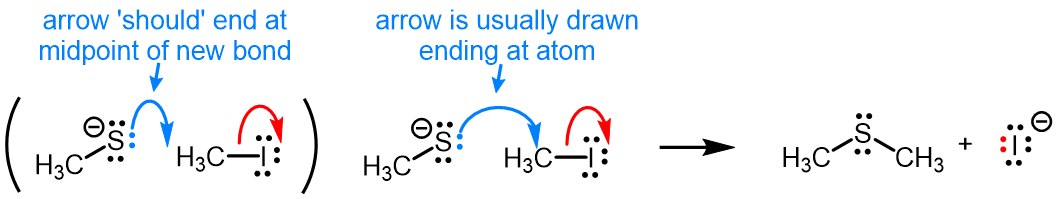
A detailed guide to drawing curved arrows is included, and the feedback given for incorrect answers to problems will guide you to the correct solutions.
Prequisite learning:
- Lewis and line structures (ChemInteractive page)
- Introduction to use of curved arrows
Learning Objectives:
- Use curved arrows to represent electron movement.
- Draw curved arrow mechanisms for common reactions.
Free Learning Resources
OrgChem101 (Flynn, Ottowa) Acid-base Reactions and Organic Mechanisms
Mechanism Inspector (Royal Society of Chemistry)
Organic Chemistry I (Morsch et al.) Using Curved Arrows in Polar Reaction Mechanisms